Surfactants are among the most versatile chemical industrial products, found in products as diverse as the motor oils we use in our cars, the medicines we take when we are sick, the detergents we use in cleaning our laundry and our homes, the drilling muds used in petroleum prospecting, and the flotation agents used in ore beneficiation. The uses of surfactants have expanded in recent decades, which include electronic printing, magnetic recording, biotechnology, microelectronics and research.
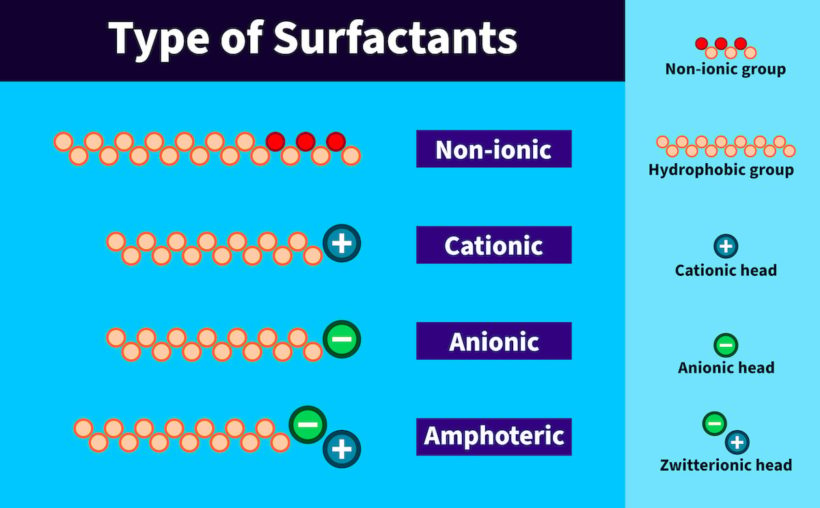
A surfactant can be thought of as a molecule with both lyophilic and lyophobic components. The lyophilic moiety is soluble in a particular fluid, whereas the lyophobic moiety is not. When the fluid is water, which is most of the time, the phrases “hydrophilic” and “hydrophobic” portions are used. The terms “amphiphile” or “amphiphilic compound” are frequently used interchangeably with “surfactant.” Amphiphilic chemicals are also abundant in nature. Surface-active chemicals are found in all biological systems. However, these compounds are more commonly referred to as “polar lipids” than “surfactants.” Thus, the name implies that a surfactant is a man-made product.
Generalisations of Surfactants
Anions are often incompatible with cations; that is, they precipitate each other from an aqueous solution unless they have water-soluble groups in their hydrophilic heads in addition to their charges. Carboxylic acid salts are more sensitive to low pH, polyvalent cations, and inert electrolytes in the aqueous phase than organic phosphoric acid salts, which are more sensitive than organic sulphates or sulfonates. All surfactants have at least one polar headgroup that prefers to be in the water and at least one hydrophobic tail that prefers to be in an apolar environment; hence, the tendency to go to interfaces.
Branched-chain unsaturated or ring-containing surfactants are more soluble in water and hydrocarbons, and have lower viscosity in aqueous media than straight-chain materials with the same number of carbon atoms; the latter is much more biodegradable but toxic to marine organisms. Amides are less susceptible to hydrolysis by hot acids or alkalis than organic sulphates or esters. Even straight fluorocarbon chains are resistant to biodegradation. Hot acids easily hydrolyse organic sulphates, while hot alkali easily hydrolyses esters.
Application of Surfactants
Surfactants are used in nearly every chemical sector, including detergents, paints, dyes, cosmetics, medicines, agrochemicals, textiles and plastics. Furthermore, surfactants have an important function in the oil industry, such as in secondary and tertiary oil recovery. Occasionally, they are also used for environmental protection, such as in oil slick dispersants. As a result, most industrial chemists require a fundamental grasp of the physical chemistry of surface-active substances, their unique features, and their phase behaviour.
Surfactants are not pure chemicals, and there can be significant variances within each chemical group. This makes sense, given those surfactants are made from various feedstocks, including petrochemicals, natural vegetable oils, and natural animal fats. Furthermore, an understanding of the fundamental phenomena involved in the application of surfactants, such as the preparation of emulsions and suspensions and their subsequent stabilisation, microemulsions, wetting spreading, adhesion, and so on, is critical in arriving at the correct composition and control of the system involved.